What About Water?
We all grew up hearing how important water was, but did anyone actually know how important it truly was. Water is around us all the time and it even makes up 70% of your body. Water is not only important to humans, but to plants and animals as well. Water is made from a covalent bond (the formation of two nonmetals bonding) of hydrogen atoms and oxygen atoms. Knowing what water is made up of helps understand how water is so versatile.

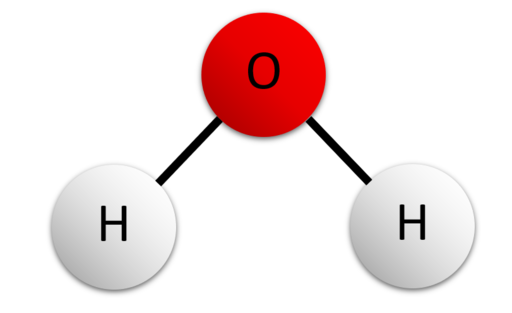
The oxygen atoms in water are bigger than the hydrogen atoms so more substances bond to it. Since opposites attract, hydrogen is positive that bonds with a negative oxygen. Oxygen and hydrogen can bond with other molecules along and similar molecules, which is important in the first property of water that will be discussed.
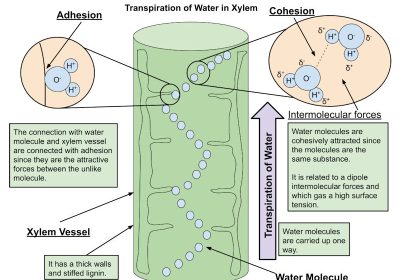
Since hydrogen is positive it bonds with negative molecules, oxygen is the opposite. Oxygen is bigger than hydrogen so its bonds are stronger. Hydrogen can bond with itself which makes water able to go against gravity- this is called cohesion. Cohesion gives water the ability to have a very strong surface that’s hard to break so some bugs can crawl on it! Cohesion has a property that does the opposite of it- adhesion. Adhesion has water going up against gravity, but adhesion is when water bonds with other molecules.
Transpiration of water in xylem is the movement of water in plant cells. It is an example of cohesion and adhesion.

The next property is specific heat. Water serves as a regulator of temperature for all forms of life. Specific heat is defined as the amount of heat needed to raise the temperature by 1 degree. Water’s high specific heat allows for the sun to be constantly beating on the surface and the heat of water to only increase by about a few degrees. The same applies at night. This allows the fish and sea life not to get too hot during the warm summer days as well as keeps temperatures low on land.
The next property that is special to water is how it is a universal solvent. Water usually dissolves most solutes it comes in contact with. This is because of how water is structured as a polar molecule. While talking about how water is a solvent, we can talk about molecules that love water and ones that hate water. Water loving molecules are called hydrophilic and molecules that hate water are hydrophobic.

Close-up of Drop on Surface by Quang Nguyen Vinh, via Pexels

The last property of water is its density as a solid. Picture a lake in the winter with a layer of ice on top of it. What you see is a solid floating on top of a liquid. Water is less dense as ice than it is as a liquid. Less dense materials float on top of more dense materials. When water freezes it becomes crystalized with its molecules equally distant from each other and less dense than liquid water. That is why ice floats on top of the lake.

Handout
Strengthen your understanding of the importance of water and it’s properties by matching the correct pairs from each column. This exercise will show you how much you’ve learned and where are your knowledge gaps about water’s properties.
Pronunciation Key and Vocabulary List
Cohesion-koh-hee-zhuhn Objects sticking to the same objects
Adhesion-ad-hee-zhuhn Objects bonding to other objects
Specific Heat-
Covalent– The sharing of electrons between atoms
Hydrogen– Colorless gas that bonds with oxygen
Oxygen– Gaseous element